The best Q10?
Differences in Q10 preparations
One of the major challenges when making Q10 supplements is dealing with the low absorption of exogenous Q10 in the digestive tract. Unprocessed Q10 is made up of crystals that need to be broken down into individual molecules before they can be absorbed. As these crystals melt only at 48 degrees Celsius or above, it is obvious that they do not dissolve at body temperature. Each Q10 molecule is also quite large and needs to be absorbed with fat.
This is why you will get low bioavailability in Q10 products made of unprocessed crystals as only very little of the crystalline Q10 will be absorbed. This is also probably a major reason why we see such a large difference in price in different Q10 products as the high-cost processing of the Q10 raw material may vary considerably.
Why bioavailability matters
Bioavailability measures the body's ability to access a substance. In order to benefit from a supplement, the active substance needs to be absorbed and made available to the body. If a supplement has a low bioavailability, it will simply pass right through the digestive system and be expelled from the body without any effect.
There are a number of different parameters that indicate that a Q10 product has a good quality and, thus, a high absorption.
High quality Q10 raw material is usually fermented from yeast. The molecular structure of this Q10 is identical to the Q10 found in the body. Unlike bacterial fermentation, yeast fermentation does not contain unknown impurities. It is also free of the dangerous cis-isomers found in low-quality synthetic Q10, which is normally produced from tobacco byproducts.
Absorption at body temperature
Unprocessed Q10 is made up of crystals that can be dissolved only in fat at a temperature of 48 degrees Celsius. This is why you get low bioavailability in many Q10 products. Less than 1% of crystalline Q10 is absorbed. It takes significant effort in the product development to increase the bioavailability of the product so that the Q10 becomes absorbable at body temperature.
The best documented bioavailability is obtained with pre-treated ubiquinone dissolved in vegetable oil in light shielding soft gelatin capsules able to dissolve completely into single Q10 molecules at body temperature. Scientific research has confirmed that the kind of Q10 that later was used in the Q-symbio study increases Q10 levels in both blood and tissue.
| Food Grade Q10 Preparations | Pharmaceutical Grade Q10 Preparations |
|---|---|
|
No specific requirements regarding variation |
High degree of uniformity from batch to batch |
| May contain some amount of impurities | Contains no impurities of unknown nature |
| Lower production costs | Higher production costs |
| No requirement for absorption | Requirements for absorption |
The crystal problem and the solution
Several clinical trials have shown that a formulation of Q10 in the form of ubiquinone dissolved in vegetable oil and delivered in light-protected soft-gelatin capsules is superior to other forms of delivery. This kind of Q10 is used in most scientific studies including the Q-symbio study since this type Q10 comes with the best documentation.
From this discussion of vegetable oil and soft-gelatin capsules, you might get the impression that there is an easy solution to the problem of poor bioavailability from Q10 crystals, but that, however, is not the case.
It is not sufficient to have a Q10 raw material of high quality and to put it in a vegetable oil. You still get a low bioavailability as the crystals do not dissolve into separate molecules at body temperature.
However, a solution to the crystal problem has been found. It consists of a special heat-pretreatment of the Q10 crystals, which alters their structure and increases their surface area so that their melting point is lowered from 48 to 37 degrees Celsius. When you consume capsules with this pretreated Q10, the Q10 content will dissolve completely in the oil, which can then easily be absorbed in the intestine like other fats.
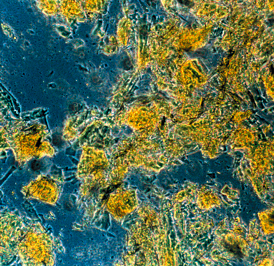 |
Here you see Q10, that has not been subjected to any pre-treatment. The crystal form prevents an efficient uptake from the intestine. |
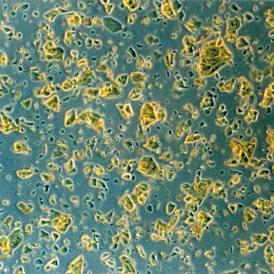 |
An example of Q10-crystals mixed with oil. You still get a poor absorption of the substance. |
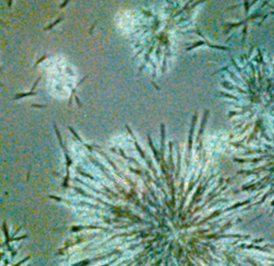 |
This is an enlarged picture of Q10 as ubiquinone that has undergone a special heat pretreatment, which has changed the crystal structure to something similar to "snowflakes" and at the same time has increased the surface area of the Q10 raw material compared to the crystals. |
.png) |
These "snowflakes" will dissolve completely in the oil at body temperature and will, thereby, release the individual Q10 molecules making the Q10 easily absorbable in the intestine. |
GMP
Products manufactured in accordance with Good Manufacturing Practice (GMP) is fine, but basically GMP is just a protocol where the manufacturer produces a set of written instructions for everything that may affect the product. Every step of the production is fully under control. Throughout the mixing, packaging, and shipping process, quality control checks ensure that the exact content of every single capsule is known when it reaches the consumer. The production is monitored by the local health authorities.
However, GMP does not regulate poorly formulated products. One can produce low quality ingredients under GMP and thus inferior products, but in a predefined, uniform quality.
Pharmaceutical standard
Products produced according to pharmaceutical standards and products that comes with proven bioavailability, efficacy and safety in controlled studies are among the best guarantee a consumer could want.
Safety profile
Q10 is generally considered a very safe substance for ingestion. Clinical studies have been conducted with dosages of Q10 ranging up to 1500 mg/day without the registration of any serious adverse effects

The body's cells can synthesise a certain amount of Q10 in the oxidized form ubiquinone, which is subsequently reduced to ubiquinol as needed. However, studies show that the natural Q10 production of the body starts to decline in the early twenties.
LINKS TO MORE INFO ON COENZYME Q10
- Dailymail: http://www.dailymail.co.uk/health/article-2331481/The-energy-boosting-supplement-halve-number-deaths-heart-failure.html
- Emaxhealth: http://www.emaxhealth.com/1506/could-antioxidant-supplement-cut-heart-failure-risk
- ESC: https://www.escardio.org/The-ESC/Press-Office/Press-releases/First-drug-to-improve-heart-failure-mortality-in-over-a-decade
- JACC: http://heartfailure.onlinejacc.org/article.aspx?articleid=1911013
- Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/25282031
- Q10facts: http://www.q10facts.com/
- Sciencedaily: http://www.sciencedaily.com/130525143852.htm
- Huffington Post: http://www.huffingtonpost.com/joel-kahn-md/heart-failure-new-hope-wi_b_9655206.html
- Morpeth Herald: https://www.morpethherald.co.uk/news/health/a-supplement-for-statin-users-1-8126041
- Holistic Primary Care: https://www.holisticprimarycare.net/latest-articles/1902-understanding-the-two-forms-of-coq10.html
- Nutrition: https://www.sciencedirect.com/science/article/pii/S089990071830488X